ATMP (Advanced Therapy Medicinal Products) are medicines for human use that are based on genes, tissues or cells and offer groundbreaking new opportunities for the treatment of disease and injury characterized by a high unmet need.
ATMPs can be classified into three main types:
- gene therapy medicines: these contain genes that lead to a therapeutic, prophylactic or diagnostic effect. They work by inserting ‘recombinant’ genes into the body, usually to treat a variety of diseases, including genetic disorders, cancer or long-term diseases. A recombinant gene is a stretch of DNA that is created in the laboratory, bringing together DNA from different sources;
- somatic-cell therapy medicines: these contain cells or tissues that have been manipulated to change their biological characteristics or cells or tissues not intended to be used for the same essential functions in the body. They can be used to cure, diagnose or prevent diseases;
- tissue-engineered medicines: these contain cells or tissues that have been modified so they can be used to repair, regenerate or replace human tissue.
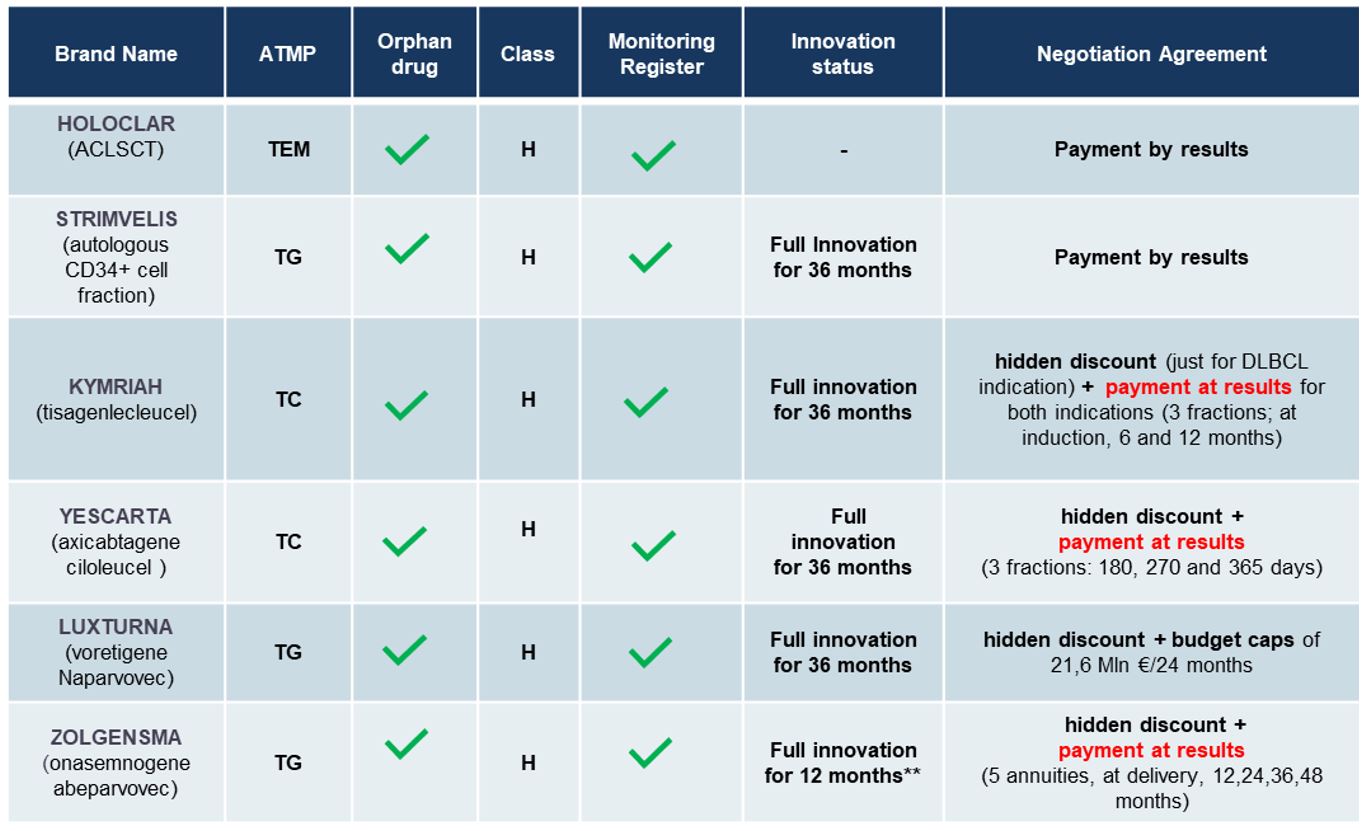
In Italy, 6 ATMPs are reimbursed by NHS (Holoclar, Strimvelis Kymriah, Yescarta, Luxturna and Zolgensma).
- all of them are Orphan Drugs:
- all negotiated compulsory discounts and MEAs (Manage Entry Agreements):
- in particular the payment at results (PaR) (annuities payment) is the most used (3/6)
- followed by payment by results (PbR – 2/6 – Holoclar and Strimvelis) and budget caps (1/6 – Luxturna)
- all received full innovation designation for 36 months, even if the quality of evidence was considered low/very low or moderate by AIFA
- all of them classified in class H and monitored by an AIFA registry
- The ex-factory price of these ATMPs varies in a range 86,000 € (Holoclar) – 2,000,000 € (Zolgensma).
First time the Italian Agency discloses an ICER that can be considered as ultimately “acceptable” for ATMPs, as a result of P&R negotiation. Never done before for any other medicinal product of any kind. The ICER threshold varies from 33.000€/QALY to 61.000€/QALY.
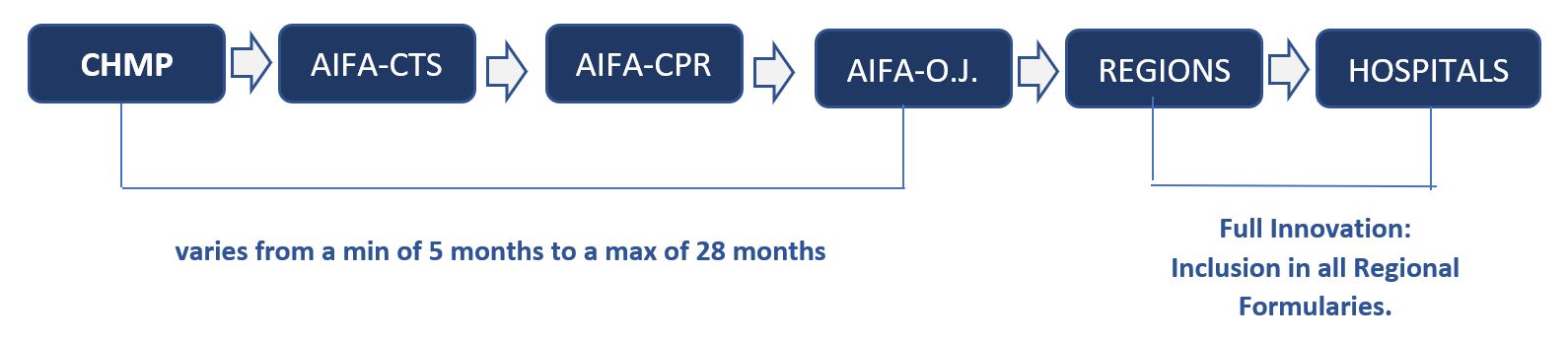
The time to access, reimbursement and negotiation (CHMP Opinion – Italian O.J. publication) for ATMPs varies from a min of 5 months to a max of 28 months.
Pricing and Reimbursement Process in Italy
Status of ATMPs in Italy